Explodable 3D Dog Skull for Veterinary Education
3D models of a Sheep and Goat Skull and Inner ear
3D models of Miocene vertebrates from Tavers
3D GM dataset of bird skeletal variation
Skeletal embryonic development in the catshark
Bony connexions of the petrosal bone of extant hippos
bony labyrinth (11) , inner ear (10) , Eocene (8) , South America (8) , Paleobiogeography (7) , skull (7) , phylogeny (6)
Lionel Hautier (23) , Maëva Judith Orliac (21) , Laurent Marivaux (16) , Rodolphe Tabuce (14) , Bastien Mennecart (13) , Renaud Lebrun (12) , Pierre-Olivier Antoine (12)
MorphoMuseuM Volume 07, issue 01
<< prev. article next article >>

|
Original articleA 3D geometric morphometric dataset quantifying skeletal variation in birdsAlexander Bjarnason
Published online: 09/02/2021 |

|
M3#5613D model of the left carpometacarpus of the superb lyrebird, Menura novaehollandia (displayed as a mirror image in the 3DHOP viewer). Type: "3D_surfaces"doi: 10.18563/m3.sf.561 state:published |
Download 3D surface file |

|
M3#5623D model of the mandible of the superb lyrebird, Menura novaehollandiae. Type: "3D_surfaces"doi: 10.18563/m3.sf.562 state:published |
Download 3D surface file |

|
M3#5633D model of the right coracoid of the superb lyrebird, Menura novaehollandiae. Type: "3D_surfaces"doi: 10.18563/m3.sf.563 state:published |
Download 3D surface file |

|
M3#5643D model of the right scapula of the superb lyrebird, Menura novaehollandiae. Type: "3D_surfaces"doi: 10.18563/m3.sf.564 state:published |
Download 3D surface file |

|
M3#5653D model of the right tarsometatarsus of the superb lyrebird, Menura novaehollandiae. Type: "3D_surfaces"doi: 10.18563/m3.sf.565 state:published |
Download 3D surface file |

|
M3#5663D model of the sternum of the superb lyrebird, Menura novaehollandiae. Type: "3D_surfaces"doi: 10.18563/m3.sf.566 state:published |
Download 3D surface file |
|
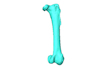
M3#5673D model of the left femur of the superb lyrebird, Menura novaehollandiae (displayed as a mirror image in the 3DHOP viewer). Type: "3D_surfaces"doi: 10.18563/m3.sf.567 state:published |
Download 3D surface file |

|
M3#5683D model of the skull of the superb lyrebird, Menura novaehollandiae. Type: "3D_surfaces"doi: 10.18563/m3.sf.568 state:published |
Download 3D surface file |

|
M3#5693D model of the left humerus of the superb lyrebird, Menura novaehollandiae (displayed as a mirror image in the 3DHOP viewer). Type: "3D_surfaces"doi: 10.18563/m3.sf.569 state:published |
Download 3D surface file |

|
M3#5703D model of the synsacrum of the superb lyrebird, Menura novaehollandiae. Type: "3D_surfaces"doi: 10.18563/m3.sf.570 state:published |
Download 3D surface file |

|
M3#5713D model of the left radius of the superb lyrebird, Menura novaehollandiae (displayed as a mirror image in the 3DHOP viewer). Type: "3D_surfaces"doi: 10.18563/m3.sf.571 state:published |
Download 3D surface file |

|
M3#5723D model of the left tibiotarsus of the superb lyrebird, Menura novaehollandiae (displayed as a mirror image in the 3DHOP viewer). Type: "3D_surfaces"doi: 10.18563/m3.sf.572 state:published |
Download 3D surface file |

|
M3#5733D model of the left ulna of the superb lyrebird, Menura novaehollandiae (displayed as a mirror image in the 3DHOP viewer). Type: "3D_surfaces"doi: 10.18563/m3.sf.573 state:published |
Download 3D surface file |
Adams D.C., Collyer M.L., 2019. Phylogenetic Comparative Methods and the Evolution of Multivariate Phenotypes. Annual Review of Ecology, Evolution, and Systematics 50, 405–425. https://doi.org/10.1146/annurev-ecolsys-110218-024555
Adams D. C., Rohlf F. J., Slice D. E., 2004. Geometric morphometrics: ten years of progress following the ‘revolution.’ Italian Journal of Zoology 71, 5–16. https://doi.org/10.1080/11250000409356545
Adams D. C., Rohlf F. J., Slice D. E., 2013. A field comes of age: geometric morphometrics in the 21st century. Hystrix 24, 7–14. https://doi.org/10.4404/hystrix-24.1-6283
Alroy J., 1998. Cope’s rule and the dynamics of body mass evolution in North American fossil mammals. Science 280, 731–734. https://doi.org/10.1126/science.280.5364.731
Arbour J.H., Curtis A.A., Santana S.E., 2019. Signatures of echolocation and dietary ecology in the adaptive evolution of skull shape in bats. Nature Communications 10, 1–13. https://doi.org/10.1038/s41467-019-09951-y
Arnold P., Amson E., Fischer M.S., 2017. Differential scaling patterns of vertebrae and the evolution of neck length in mammals. Evolution 71, 1587–1599. https://doi.org/10.1111/evo.13232
Bardua C., Felice R.N., Watanabe A., Fabre A.C., Goswami A., 2019a. A practical guide to sliding and surface semilandmarks in morphometric analyses. Integrative Organismal Biology 1, obz016. https://doi.org/10.1093/iob/obz016
Bardua C., Wilkinson M., Gower D. J., Sherratt E., Goswami A., 2019b. Morphological evolution and modularity of the caecilian skull. BMC Evolutionary Biology 19, 30. https://doi.org/10.1186/s12862-018-1342-7
Bardua C., Fabre A.C., Bon M., Das K., Stanley E. L., Blackburn D. C., Goswami A., 2020. Evolutionary integration of the frog cranium. Evolution 74, 6 . https://doi.org/10.1111/evo.13984
Baumel J. J., Witmer L., 1993. Osteologia; p. 45–132. In: Baumel J.J., King J.E., Breazile J.E., Evans H.E., Vanden Berge J.C., (Eds), Handbook of Avian Anatomy: Nomina Anatomica Avium, 2nd Edition. Nuttall Ornithological Club, Cambridge, pp. 45–132.
Bell E., Andres B., Goswami A., 2011. Integration and dissociation of limb elements in flying vertebrates: a comparison of pterosaurs, birds and bats. Journal of Evolutionary Biology 24, 2586–2599. https://doi.org/10.1111/j.1420-9101.2011.02381.x
Bhullar B.A.S., Marugán-Lobón J., Racimo F., Bever G.S., Rowe T.B., Norell M.A., Abzhanov A., 2012. Birds have paedomorphic dinosaur skulls. Nature 487, 223–226. https://doi.org/10.1038/nature11146
Botelho J. F., Ossa-Fuentes L., Soto-Acuña S., Smith-Paredes D., Nuñez-León D., Salinas-Saavedra M., Ruiz-Flores M., Vargas A.O., 2014. New developmental evidence clarifies the evolution of wrist bones in the dinosaur–bird transition. PLoS Biology 12, e1001957. https://doi.org/10.1371/journal.pbio.1001957
Botton-Divet L., Cornette R., Fabre A.-C., Herrel A., Houssaye A., 2016. Morphological analysis of long bones in semi-aquatic mustelids and their terrestrial relatives. Integrative and Comparative Biology 56, 1298–1309. https://doi.org/10.1093/icb/icw124
Bright J. A., Marugán-Lobón J., Cobb S.N., Rayfield E.J., 2016. The shapes of bird beaks are highly controlled by nondietary factors. Proceedings of the National Academy of Sciences 113, 5352–5357. https://doi.org/10.1073/pnas.1602683113
Cardini, A., 2016. Lost in the other half: improving accuracy in geometric morphometric analyses of one side of bilaterally symmetric structures. Systematic Biology 65, 1096-1106. https://doi.org/10.1093/sysbio/syw043
Cardini, A., 2017. Left, right or both? Estimating and improving accuracy of one‐side‐only geometric morphometric analyses of cranial variation. Journal of Zoological Systematics and Evolutionary Research 55, 1-10. https://doi.org/10.1111/jzs.12144
Cheverud J. M., 1982. Phenotypic, genetic, and environmental morphological integration in the cranium. Evolution 36, 499–516. https://doi.org/10.2307/2408096
Clark Jr G.A., 1993. Anatomia topographica externa. In: Baumel J.J., King J.E., Breazile J.E., Evans H.E., Vanden Berge J.C., (Eds), Handbook of Avian Anatomy: Nomina Anatomica Avium, 2nd Edition. Nuttall Ornithological Club, Cambridge, pp. 7–16.
Coombs E. J., Clavel J., Park T., Churchill M., Goswami A., 2020. Wonky whales: the evolution of cranial asymmetry in cetaceans. BMC Biology 18,1–24. https://doi.org/10.1186/s12915-020-00805-4
Cooney C. R., Bright J.A., Capp E.J.R., Chira A.M., Hughes E.C., Moody C.J.A., Nouri L.O., Varley Z.K., Thomas G.H., 2017. Mega-evolutionary dynamics of the adaptive radiation of birds. Nature 542, 344–347. https://doi.org/10.1038/nature21074
Cooper N., Purvis A., 2010. Body size evolution in mammals: complexity in tempo and mode. The American Naturalist 175, 727–738. https://doi.org/10.1086/652466
Corfield J. R., Price K., Iwaniuk A.N., Gutierrez-Ibañez C., Birkhead T., Wylie D.R., 2015. Diversity in olfactory bulb size in birds reflects allometry, ecology, and phylogeny. Frontiers in Neuroanatomy 9, 102. https://doi.org/10.3389/fnana.2015.00102
Davies T. G., et al. 2017. Open data and digital morphology. Proceedings of the Royal Society B: Biological Sciences 284, 20170194. https://doi.org/10.1098/rspb.2017.0194
Dececchi T.A., Larsson H.C.E., 2013. Body and limb size dissociation at the origin of birds: uncoupling allometric constraints across a macroevolutionary transition. Evolution 67, 2741–2752. https://doi.org/10.1111/evo.12150
Etienne R.S., Haegeman B., 2012. A conceptual and statistical framework for adaptive radiations with a key role for diversity dependence. The American Naturalist 180, E75–E89. https://doi.org/10.1086/667574
Ezard T.H.G., Aze T., Pearson P.N., Purvis A., 2011. Interplay between changing climate and species’ ecology drives macroevolutionary dynamics. Science 332, 349–351. https://doi.org/10.1126/science.1203060
Ezard T.H.G., Purvis A., 2016. Environmental changes define ecological limits to species richness and reveal the mode of macroevolutionary competition. Ecology Letters, 19, 899–906. https://doi.org/10.1111/ele.12626
Fabre A.-C., Bardua C., Bon M., Clavel J., Felice R.N., Streicher J.W., Bonnel J., Stanley E. L., Blackburn D. C., Goswami A., 2020. Metamorphosis shapes cranial diversity and rate of evolution in salamanders. Nature Ecology & Evolution 4, 1129–1140. https://doi.org/10.1038/s41559-020-1225-3
Fabre A.-C., Bickford D., Segall M., Herrel A., 2016. The impact of diet, habitat use, and behaviour on head shape evolution in homalopsid snakes. Biological Journal of the Linnean Society 118, 634–647. https://doi.org/10.1111/bij.12753
Fabre A.-C., Goswami A., Peigné S., Cornette R., 2014. Morphological integration in the forelimb of musteloid carnivorans. Journal of Anatomy 225, 19–30. https://doi.org/10.1111/joa.12194
Felice R.N., Goswami A., 2018. Developmental origins of mosaic evolution in the avian cranium. Proceedings of the National Academy of Sciences 115, 555–560. https://doi.org/10.1073/pnas.1716437115
Felice R.N., Tobias J.A., Pigot A.L., Goswami A., 2019. Dietary niche and the evolution of cranial morphology in birds. Proceedings of the Royal Society B 286, 20182677. https://doi.org/10.1073/pnas.1716437115
Garamszegi L.Z., 2014. Modern Phylogenetic Comparative Methods and Their Application in Evolutionary Biology: Concepts and Practice. Springer. https://doi.org/10.1007/978-3-662-43550-2
Gingerich P., 1983. Rates of evolution: effects of time and temporal scaling. Science 222, 159–162. https://doi.org/10.1126/science.222.4620.159
Goswami,A., 2006. Cranial modularity shifts during mammalian evolution. The American Naturalist 168, 270–280. https://doi.org/10.1086/505758
Goswami A., Watanabe A., Felice R.N., Bardua C., Fabre A.C., Polly P.D., 2019. High-density morphometric analysis of shape and integration: the good, the bad, and the not-really-a-problem. Integrative and Comparative Biology 59, 669–683. https://doi.org/10.1093/icb/icz120
Gower J.C., 1975. Generalized procrustes analysis. Psychometrika 40, 33–51. https://doi.org/10.1007/BF02291478
Hanot P., Herrel A., Guintard C., Cornette R., 2018. The impact of artificial selection on morphological integration in the appendicular skeleton of domestic horses. Journal of Anatomy 232, 657–673. https://doi.org/10.1111/joa.12772
Hansen T.F., 1997. Stabilizing selection and the comparative analysis of adaptation. Evolution 51, 1341–1351. https://doi.org/10.1111/j.1558-5646.1997.tb01457.x
Hansen T.F. 2012. Adaptive landscapes and macroevolutionary dynamics. In: Svennson E., Calsbeek R. (Eds), The Adaptive Landscape in Evolutionary Biology. Oxford University Press, Oxford, pp. 205-226. https://doi.org/10.1093/acprof:oso/9780199595372.003.0013
Harmon L.J., Losos J.B., Jonathan Davies T., Gillespie R.G., Gittleman J.L., Bryan Jennings W., Kozak K.H., McPeek M.A., Moreno‐Roark F., Near T.J., 2010. Early bursts of body size and shape evolution are rare in comparative data. Evolution: International Journal of Organic Evolution 64, 2385–2396. https://doi.org/10.1111/j.1558-5646.2010.01025.x
Harmon L.J., Schulte J.A., Larson A., Losos J.B., 2003. Tempo and mode of evolutionary radiation in iguanian lizards. Science 301, 961–964. https://doi.org/10.1126/science.1084786
Hautmann M., 2020. What is macroevolution? Palaeontology 63, 1–11. https://doi.org/10.1111/pala.12465
Heers A.M., Dial K.P., 2015. Wings versus legs in the avian bauplan: development and evolution of alternative locomotor strategies. Evolution 69, 305–320. https://doi.org/10.1111/evo.12576
Jablonski D., 2005. Mass extinctions and macroevolution. Paleobiology 31, 192–210. https://doi.org/10.1666/0094-8373(2005)031[0192:MEAM]2.0.CO;2
Jablonski D., 2008. Biotic interactions and macroevolution: extensions and mismatches across scales and levels. Evolution: International Journal of Organic Evolution 62, 715–739. https://doi.org/10.1111/j.1558-5646.2008.00317.x
Jarvis E.D., Mirarab S., Aberer A.J., Li B., Houde P., Li C., Ho S.Y.W., Faircloth B.C., Nabholz B., J.T. Howard., 2014. Whole-genome analyses resolve early branches in the tree of life of modern birds. Science 346, 1320–1331. https://doi.org/10.1126/science.1253451
Jetz W., Thomas G.H., Joy J.B., Hartmann K., Mooers A.O., 2012. The global diversity of birds in space and time. Nature 491, 444–448. https://doi.org/10.1038/nature11631
Klingenberg C.P., 2010. Evolution and development of shape: integrating quantitative approaches. Nature Reviews Genetics 11, 623–635. https://doi.org/10.1038/nrg2829
Klingenberg C.P., J. Marugán-Lobón., 2013. Evolutionary covariation in geometric morphometric data: analyzing integration, modularity, and allometry in a phylogenetic context. Systematic Biology 62, 591–610. https://doi.org/10.1038/nrg2829
Kulemeyer C., Asbahr K., Gunz P., Frahnert S., Bairlein F., 2009. Functional morphology and integration of corvid skulls – a 3D geometric morphometric approach. Frontiers in Zoology 6, 2. https://doi.org/10.1186/1742-9994-6-2
Livezey B.C., Zusi R.L., 2006. Higher-Order Phylogeny of Modern Birds (Theropoda, Aves: Neornithes) Based on Comparative Anatomy. 1, Methods and Characters. Carnegie Museum of Natural History 37, 1-556. https://doi.org/10.2992/0145-9058(2006)37[1:PON]2.0.CO;2
Livezey B.C., Zusi R.L., 2007. Higher-order phylogeny of modern birds (Theropoda, Aves: Neornithes) based on comparative anatomy. II. Analysis and discussion. Zoological Journal of the Linnean Society 149, 1–95. https://doi.org/10.1111/j.1096-3642.2006.00293.x
Losos J.B., Mahler D.L., 2010. Adaptive radiation: the interaction of ecological opportunity, adaptation, and speciation. In Bell MA., Futuyma D.J., Eanes W.F., Levinton J.S. (Eds.), Evolution after Darwin: the first 150 years. Sinauer, Sunderland, MA, pp. 381-420.
Michaud M., Veron G., Fabre A., 2020. Phenotypic integration in feliform carnivores: covariation patterns and disparity in hypercarnivores versus generalists. Evolution, In Press. https://doi.org/10.1111/evo.14112
Navalón G., Marugán-Lobón J., Bright J. A., Cooney C. R., Rayfield E.J., 2020. The consequences of craniofacial integration for the adaptive radiations of Darwin’s finches and Hawaiian honeycreepers. Nature Ecology & Evolution 4, 270–278. https://doi.org/10.1038/s41559-019-1092-y
Nudds R.L., Dyke G.J., Rayner J.M.V., 2007. Avian brachial index and wing kinematics: putting movement back into bones. Journal of Zoology, 272, 218–226. https://doi.org/10.1111/j.1469-7998.2006.00261.x
Oliveros C.H., Field D.J., Ksepka D.T., Barker F.K., Aleixo A., Andersen M.J., Alström P., Benz B.W., Braun E.L., Braun M.J., 2019. Earth history and the passerine superradiation. Proceedings of the National Academy of Sciences 116, 7916–7925. https://doi.org/10.1073/pnas.1813206116
Olson E.C., Miller R.L. 1952. Morphological Integration. University of Chicago Press, Chicago.
Paluh D.J., Stanley E.L., Blackburn D.C., 2020. Evolution of hyperossification expands skull diversity in frogs. Proceedings of the National Academy of Sciences 117, 8554–8562. https://doi.org/10.1073/pnas.2000872117
Pennell M.W., Harmon L.J., 2013. An integrative view of phylogenetic comparative methods: connections to population genetics, community ecology, and paleobiology. Annals of the New York Academy of Sciences, 1289, 90–105. https://doi.org/10.1111/nyas.12157
Pigot A.L., Sheard C.,. Miller E.T, Bregman T.P., Freeman B.G., Roll U., Seddon N., Trisos C.H., Weeks B.C., Tobias J.A., 2020. Macroevolutionary convergence connects morphological form to ecological function in birds. Nature Ecology & Evolution 4, 1–10. https://doi.org/10.1038/s41559-019-1070-4
Proctor N., Lynch P., 1993. Manual of Ornithology : Avian Structure and Function. Yale University Press, New Haven.
Prum R.O., Berv J.S., Dornburg A., Field D.J., Townsend J.P., Lemmon E.M., Lemmon A.R., 2015. A comprehensive phylogeny of birds (Aves) using targeted next-generation DNA sequencing. Nature 526, 569–573. https://doi.org/10.1038/nature15697
Randau M., Goswami A., 2018. Shape covariation (or the lack thereof) between vertebrae and other skeletal traits in felids: the whole is not always greater than the sum of parts. Evolutionary Biology 45, 196–210. https://doi.org/10.1007/s11692-017-9443-6
Rohlf F.J., Slice D., 1990. Extensions of the Procrustes method for the optimal superimposition of landmarks. Systematic Biology 39, 40–59. https://doi.org/10.2307/2992207
Rohlf F. J., Marcus L.F., 1993. A revolution morphometrics. Trends in Ecology & Evolution 8, 129–132. https://doi.org/10.1016/0169-5347(93)90024-J
Schluter D., 2000. The Ecology of Adaptive Radiation. Oxford University Press, Oxford. https://doi.org/10.1093/oso/9780198505235.001.0001
Schneider C.A., Rasband W.S., Eliceiri K.W., 2012. NIH Image to ImageJ: 25 years of image analysis. Nature Methods 9, 671–675. https://doi.org/10.1038/nmeth.2089
Segall M., Herrel A., Godoy-Diana R.., 2019. Hydrodynamics of frontal striking in aquatic snakes: drag, added mass, and the possible consequences for prey capture success. Bioinspiration & Biomimetics 14, 36005. https://doi.org/10.1088/1748-3190/ab0316
Segall M., Cornette R., Fabre A.-C., Godoy-Diana R., Herrel A., 2016. Does aquatic foraging impact head shape evolution in snakes? Proceedings of the Royal Society B: Biological Sciences 283, 20161645. https://doi.org/10.1098/rspb.2016.1645
Serrano F.J., Costa-Pérez M., Navalón G., Martín-Serra A., 2020. Morphological Disparity of the Humerus in Modern Birds. Diversity 12, 173. https://doi.org/10.3390/d12050173
Shatkovska O.V, Ghazali M., Mytiai I.S., Druz N., 2018. Size and shape correlation of birds’ pelvis and egg: Impact of developmental mode, habitat, and phylogeny. Journal of Morphology 279, 1590–1602. https://doi.org/10.1002/jmor.20888
Silvestro D., Antonelli A., Salamin N., Quental T.B., 2015. The role of clade competition in the diversification of North American canids. Proceedings of the National Academy of Sciences 112, 8684–8689. https://doi.org/10.1073/pnas.1502803112
Simpson G.G. 1944. Tempo and Mode in Evolution. Columbia University Press, New York.
Simpson G.G. 1953. The Major Features of Evolution. Columbia University Press, New York. https://doi.org/10.7312/simp93764
Tokita M., Yano W., James H.F., Abzhanov A., 2017. Cranial shape evolution in adaptive radiations of birds: comparative morphometrics of Darwin’s finches and Hawaiian honeycreepers. Philosophical Transactions of the Royal Society B: Biological Sciences 372, 20150481. https://doi.org/10.1098/rstb.2015.0481
Venditti C., Meade A., Pagel M., 2011. Multiple routes to mammalian diversity. Nature 479, 393–396. doi: 10.1038/nature10516. https://doi.org/10.1038/nature10516
Vermeij G.J., 1973. Biological versatility and earth history. Proceedings of the National Academy of Sciences 70, 1936–1938. https://doi.org/10.1073/pnas.70.7.1936
Vrba E.S., 1983. Macroevolutionary trends: new perspectives on the roles of adaptation and incidental effect. Science 221, 387–389. https://doi.org/10.1126/science.221.4608.387
Watanabe A., Fabre A.C., Felice R.N., Maisano J.A., Müller J., Herrel A., Goswami A., 2019. Ecomorphological diversification in squamates from conserved pattern of cranial integration. Proceedings of the National Academy of Sciences 116, 14688–14697. https://doi.org/10.1073/pnas.1820967116
Wilman H., Belmaker J., Simpson J., De La Rosa C., Rivadeneira M.M., Jetz W., 2014. EltonTraits 1.0: Species‐level foraging attributes of the world’s birds and mammals. Ecology 95, 2027. https://doi.org/10.1890/13-1917.1
Wright N.A., Steadman D.W., Witt C.C., 2016. Predictable evolution toward flightlessness in volant island birds. Proceedings of the National Academy of Sciences 113, 4765–4770. https://doi.org/10.1073/pnas.1522931113
Yoder J.B., Clancey E., Des Roches S., Eastman J.M., Gentry L., Godsoe W., Hagey T.J., Jochimsen D., Oswald B.P., Robertson J., 2010. Ecological opportunity and the origin of adaptive radiations. Journal of Evolutionary Biology 23, 1581–1596. https://doi.org/10.1111/j.1420-9101.2010.02029.x
Zusi R.L., 1993. Patterns of Diversity in the Avian Skull. In: Hanken J., Hall B.K. (Eds.), The skull. Vol. 2. The University of Chicago Press, Chicago, pp. 391–437.
Andrew Orkney, Alex Bjarnason, Brigit C. Tronrud and Roger B. J. Benson (2021). Patterns of skeletal integration in birds reveal that adaptation of element shapes enables coordinated evolution between anatomical modules. Nature Ecology & Evolution. https://doi.org/10.1038/s41559-021-01509-w
Daniel Rhoda, Marion Segall, Olivier Larouche, Kory Evans and Kenneth D Angielczyk (2021). Local Superimpositions Facilitate Morphometric Analysis of Complex Articulating Structures. Integrative and Comparative Biology. https://doi.org/10.1093/icb/icab031
Christopher T. Griffin, João F. Botelho, Michael Hanson, Matteo Fabbri, Daniel Smith-Paredes, Ryan M. Carney, Mark A. Norell, Shiro Egawa, Stephen M. Gatesy, Timothy B. Rowe, Ruth M. Elsey, Sterling J. Nesbitt and Bhart-Anjan S. Bhullar (2022). The developing bird pelvis passes through ancestral dinosaurian conditions. Nature. https://doi.org/10.1038/s41586-022-04982-w
Jesús Marugán‐Lobón, Sergio M. Nebreda, Guillermo Navalón and Roger B. J. Benson (2022). Beyond the beak: Brain size and allometry in avian craniofacial evolution. Journal of Anatomy. https://doi.org/10.1111/joa.13555
Emma J. Holvast and Daniel B. Thomas (2022). Taxonomic classification of seabird long bones using 3D shape: A method with wider potential in zooarchaeology. Journal of Archaeological Science: Reports. https://doi.org/10.1016/j.jasrep.2022.103641
Juan Benito, Pei-Chen Kuo, Klara E. Widrig, John W. M. Jagt and Daniel J. Field (2022). Cretaceous ornithurine supports a neognathous crown bird ancestor. Nature. https://doi.org/10.1038/s41586-022-05445-y
Guillermo Navalón, Alexander Bjarnason, Elizabeth Griffiths and Roger B. J. Benson (2022). Environmental signal in the evolutionary diversification of bird skeletons. Nature. https://doi.org/10.1038/s41586-022-05372-y
Case Vincent Miller, Michael Pittman, Xiaoli Wang, Xiaoting Zheng and Jen A. Bright (2022). Diet of Mesozoic toothed birds (Longipterygidae) inferred from quantitative analysis of extant avian diet proxies. BMC Biology. https://doi.org/10.1186/s12915-022-01294-3
Han Hu, Yan Wang, Paul G McDonald, Stephen Wroe, Jingmai K O'Connor, Alexander Bjarnason, Joseph J Bevitt, Xuwei Yin, Xiaoting Zheng, Zhonghe Zhou and Roger BJ Benson (2022). Earliest evidence for fruit consumption and potential seed dispersal by birds. eLife. https://doi.org/10.7554/eLife.74751
Aubrey Keirnan, Trevor H. Worthy, Jeroen B. Smaers, Karine Mardon, Andrew N. Iwaniuk and Vera Weisbecker (2022). Not like night and day: the nocturnal letter-winged kite does not differ from diurnal congeners in orbit or endocast morphology. Royal Society Open Science. https://doi.org/10.1098/rsos.220135
Talia M. Lowi-Merri, Oliver E. Demuth, Juan Benito, Daniel J. Field, Roger B. J. Benson, Santiago Claramunt and David C. Evans (2023).
Reconstructing locomotor ecology of extinct avialans: a case study of
Ichthyornis
comparing sternum morphology and skeletal proportions
. Proceedings of the Royal Society B: Biological Sciences. https://doi.org/10.1098/rspb.2022.2020
Alexander D. Clark, Han Hu, Roger BJ Benson and Jingmai K. O’Connor (2023). Reconstructing the dietary habits and trophic positions of the Longipterygidae (Aves: Enantiornithes) using neontological and comparative morphological methods. PeerJ. https://doi.org/10.7717/peerj.15139
Pei‐Chen Kuo, Roger B. J. Benson and Daniel J. Field (2023). The influence of fossils in macroevolutionary analyses of 3D geometric morphometric data: A case study of galloanseran quadrates. Journal of Morphology. https://doi.org/10.1002/jmor.21594
Martin Segesdi, Delphine Brabant, Raphaël Cornette and Alexandra Houssaye (2024). How does the shape of the wing and hindlimb bones of aquatic birds relate to their locomotor abilities?. The Anatomical Record. https://doi.org/10.1002/ar.25512
Enzo M.R. Reyes, Simone Giovanardi, Giovanny Suarez-Espin, Ben Haase, Kalinka Rexer-Huber, Graham Parker, Paul Sagar and Johannes, H. Fischer (2024). Where do some Aotearoa New Zealand seabirds go? Records of Thalassarche albatrosses and Procellaria petrels in Ecuadorian waters. Notornis. https://doi.org/10.63172/894732bkgvia
Nicoleta Manuta, Buket Çakar, Ozan Gündemir and Mihaela-Claudia Spataru (2024). Shape and Size Variations of Distal Phalanges in Cattle. Animals. https://doi.org/10.3390/ani14020194
Pei-Chen Kuo, Guillermo Navalón, Roger B. J. Benson and Daniel J. Field (2024). Macroevolutionary drivers of morphological disparity in the avian quadrate. Proceedings of the Royal Society B: Biological Sciences. https://doi.org/10.1098/rspb.2023.2250
Ashleigh Haruda, Camilla Mazzucato and Lisa Yeomans (2024). On the wing: Morphological variation in the osteology of Mediterranean, Near Eastern, and European Anatidae (excluding Anserinae). Journal of Morphology. https://doi.org/10.1002/jmor.21750
Talia M. Lowi-Merri, Martina Gjevori, Zbigniew M. Bochenski, Krzysztof Wertz and Santiago Claramunt (2024). Total-evidence dating and the phylogenetic affinities of early fossil passerines. Journal of Systematic Palaeontology. https://doi.org/10.1080/14772019.2024.2356086
Luis M. Chiappe, Guillermo Navalón, Agustín G. Martinelli, Ismar de Souza Carvalho, Rodrigo Miloni Santucci, Yun-Hsin Wu and Daniel J. Field (2024). Cretaceous bird from Brazil informs the evolution of the avian skull and brain. Nature. https://doi.org/10.1038/s41586-024-08114-4
Sergio Almécija, Kelsey D. Pugh, Alisha Anaya, Christopher M. Smith, Nancy B. Simmons, Robert S. Voss, Neil Duncan, Darrin P. Lunde, Megan K. Viera, Teresa Hsu, Emmanuel Gilissen, Stephanie A. Maiolino, Julie M. Winchester, Biren A. Patel, Caley M. Orr, Matthew W. Tocheri, Eric Delson, Ashley S. Hammond, Doug M. Boyer and Santiago A. Catalano (2024). Primate Phenotypes: A Multi-Institution Collection of 3D Morphological Data Housed in MorphoSource. Scientific Data. https://doi.org/10.1038/s41597-024-04261-5
Andrew Orkney, David B. Boerma and Brandon P. Hedrick (2024). Evolutionary integration of forelimb and hindlimb proportions within the bat wing membrane inhibits ecological adaptation. Nature Ecology & Evolution. https://doi.org/10.1038/s41559-024-02572-9
Klara Widrig, Fabio Alfieri, Pei-Chen Kuo, Helen James and Daniel J. Field (2025). Quantitative analysis of stem-palaeognath flight capabilities sheds light on ratite dispersal and flight loss. Biology Letters. https://doi.org/10.1098/rsbl.2025.0320
Andrew Orkney, Priscila S. Rothier and Brandon P. Hedrick (2025). Great expectations: altricial developmental strategies are associated with more flexible evolution of limb skeleton proportions in birds. Proceedings of the Royal Society B: Biological Sciences. https://doi.org/10.1098/rspb.2025.1647
Idriss Pelletan, Raphaël Cornette and Anick Abourachid (2025). Ecomorphological Analysis of the Bird Lumbosacral Organ in an Evolutionary Context. Journal of Morphology. https://doi.org/10.1002/jmor.70073
Trevor H. Worthy, R. Paul Scofield, Suzanne J. Hand, Michael Archer and Vanesa L. De Pietri (2025). A large cracticine passerine (Aves, Artamidae, Cracticinae) from the Early Miocene, St Bathans Fauna of New Zealand. PalZ. https://doi.org/10.1007/s12542-025-00736-x
Fabio Alfieri, Oliver E. Demuth, Elizabeth M. Steell, Anne‐Claire Fabre and Daniel J. Field (2025). Constrained variation in the internal architecture of avian wing bones. Functional Ecology. https://doi.org/10.1111/1365-2435.70178
Edson Guilherme, Ighor D. Mendes, Carlos D’Apolito, Lucy G. Souza, Francisco R. Negri, Kathellen G. Magalhães and Jonas P. Souza-Filho (2025). The tibiotarsus of a giant darter from the upper Miocene of Amazonia and weight estimates for fossil darters. Palaeoworld. https://doi.org/10.1016/j.palwor.2024.10.003
Elizabeth M. Steell, Daniel J. Field, Pascale Lubbe, Alex Brown, Nicolas J. Rawlence and Alan J.D. Tennyson (2025). A possible early bowerbird from the Miocene of New Zealand. Historical Biology. https://doi.org/10.1080/08912963.2025.2568099
A Chen, E M Steell, R B J Benson and D J Field (2025). Toward a Comprehensive Anatomical Matrix for Crown Birds: Phylogenetic Insights from the Pectoral Girdle and Forelimb Skeleton. Integrative Organismal Biology. https://doi.org/10.1093/iob/obaf029
Kathleen L.S. Garland, Eleanor M. Hay, Daniel J. Field and Alistair R. Evans (2025). Common developmental origins of beak shapes and evolution in theropods. iScience. https://doi.org/10.1016/j.isci.2025.112246